NCERT Textual Problems Unit-7-B
Q.7.27
Monochromatic radiation of wavelength 640.2nm (1 nm = 10-9 m) from a neon lamp irradiates a photosensitive material made of cesium on tungsten. The stopping voltage is measured to be 0.54 V. The source is replaced with an iron source, and its 427nm line irradiates the same photocell. Predict the new stopping voltage.
NCERT Textual Problems Unit-7-B
Answer:
For neon lamps: λ = 640.2 nm = 640.2⨯10-9 m and V0 = 0.54 V Now, h v = h vo +1/2 m v2max or h c /λ = ω + e V0 or ω = ( h c /λ ) – e Vo
= (6.62⨯10-34 ⨯ 3⨯108 )/ 640.2⨯10-9 -1.6⨯10-19 ⨯ 0.54
= 3.102⨯10-19 – 0.864⨯10-19 m ;
= 2.238⨯10-19 J
NCERT Textual Problems Unit-7-B
For the iron source: λ = 427.2 nm = 427.2⨯10-9 m ; and ω = 2.238⨯10-19 J Again, ω = ( h c /λ ) – e V0 e V0 = ( h c /λ ) – ω V0 = 1/e [ ( h c /λ) – ω] = 1/1.6⨯10-19 ⨯[ (6.62⨯10-34 ⨯ 3⨯108 )/ 427.2⨯10-9 ] – 2.238⨯10-19 = 1/1.6⨯10-19 (4.649 ⨯10-19 – 2.238⨯10-19) = 2.411 ⨯10-19/ 1.6⨯10-19 = 1.507 V
Q.7.28
A mercury lamp is a convenient source for studying the frequency dependence of photoelectric emission since it gives a number of spectral lines ranging from the UV to the red end of the visible spectrum. In our experiment with a Rubidium photocell, the following lines from a mercury source were used:
NCERT Textual Problems Unit-7-B
λ1 = 3,650 Å, λ2 = 4,047 Å, λ3 = 4,358 Å, λ4 = 5,461 Å, λ5 = 6,907 Å
The stopping voltages, respectively, were measured to be:
V01 = 1.28 V, V02 = 0.95 V, V03 = 0.74 V, V04 = 0.16 V, and V05 = 0 V
(a) Determine the value of Planck’s constant h
(b) Estimate the threshold frequency and work function for the material. NCERT Textual Problems Unit-7-B
Solution:
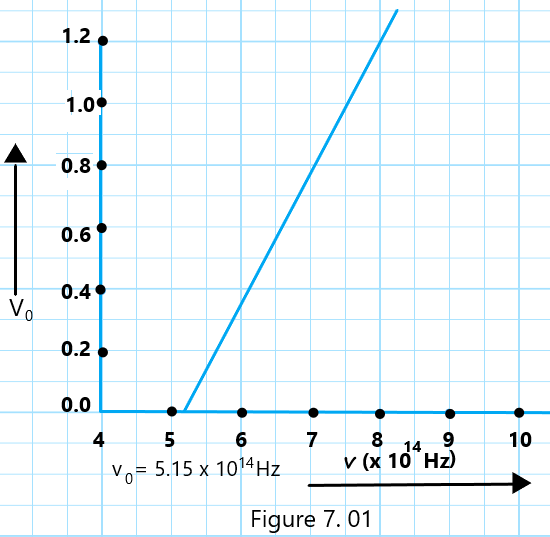
(a) Let the respective frequencies of the five spectral lines of Mercury be v1, v2, v3, v4, and v5. then,
v1 = c/λ1= 3⨯108 /3,650 ⨯10-10 = 8.22⨯1014 Hz, v2 = c/λ2 = 3⨯108/4,047⨯10-10 = 7.41⨯1014 Hz, v3= c/λ3 = 3⨯108/4,358⨯10-10 = 6.88⨯1014 Hz, v4 = c/λ4 = 3⨯108/ 5,461⨯10-10 = 5.49⨯1014 Hz, and v5 = c/λ4 = 3⨯108/6,907⨯10-10 =4.34⨯1014 Hz,
We can calculate h v from Einstein’s photoelectric equation: h v = h v0 + 1/2 m vmax2
If V0 represents the stopping potential, e V0 = 1/2 m vmax2 As a result, h v = h V0 + e V0, or V0 = h v/e – h V0/e.
It represents the equation of a straight line, whose slope is h/e, and makes an intercept hV0/e on the negative V0 axis. The plot of the graph between v (along the X-axis) and V0 (along the y-axis) for the given data for the five spectral lines of mercury will be shown in Fig. 7.01.
(b) Also, the intercept made by the graph on the v-axis is equal to v0. Therefore, from the graph, we have v0 = 5.15⨯1014 Hz
Hence, the work function of rubidium, ω = h v0 = 6.56⨯10-34⨯5.15⨯1014 = 3.38⨯10-19 J = 3.38⨯10-19/1.6⨯10-19 = 2.11 eV
NCERT Textual Problems Unit-7-B
Q.7.29
The work function for the following metals is given: Na : 2.75 eV ; K : 2.30 eV ; Mo : 4.17 eV ; Ni : 5.15 eV
Which of these metals will not give photoelectric emission for radiation of wavelength 3,300 Å from a He-Cd laser placed 1 m away from the photocell? What happens if the laser is brought nearer and placed 50 cm away?
NCERT Textual Problems Unit-7-B
Solution:
Here, λ = 3,300 Å = 3,300⨯10-10 m
Therefore, the energy of a photon of incident light,
E = h c /λ = (6.62⨯10-34 ⨯ 3⨯108 ) / 3,300⨯10-10
6.018 ⨯10-19 J = 6.018 ⨯10-19/1.6⨯10-19 = 3.76 eV
Since the work functions of Mo and Ni are greater than the energy of the photon of the incident light, photoelectric emission will not occur for these metals.
If the laser is brought nearer, the intensity of the incident radiation on the metals will increase. It will increase the photoemission in the cases of Na and K. However, no photoelectric emission will take place in the case of Mo and Ni.
NCERT Textual Problems Unit-7-B
Q.7.30
Light of intensity 10 -5 W m -2 falls on a sodium photocell of a surface area of 2 cm2. Assuming that the top 5 layers of sodium absorb the incident energy, estimate the time required for photoelectric emission in the wave picture of radiation. The work function for the metal is given to be 2 eV. What is the implication of your answer? NCERT Textual Problems Unit-7-B
Solution:
Since the size of the atom is about 10-10 m, the effective area of an atom may be considered as 10 -20 m2 (roughly). The area of each layer in sodium = 2 cm2 = 2⨯10-4 m2 . Therefore, the number of atoms in the 5 Layer of sodium = (2⨯10-4 ⨯ 5 )/ 10-20 = 1017 If we assume that sodium has one conduction electron per atom, then the number of electrons in the 5 layers of sodium is 1017
Intensity of incident light = 10-5 W/m2. Therefore, energy incident per second on the surface of the photocell = 10-5⨯ 2⨯10-4 = 2⨯10-9 W
Since the energy is absorbed by 5 layers of sodium, according to the wave picture of radiation, the electrons present in all 5 layers of sodium will share the incident energy equally. Therefore, energy received by any one electron in the 5 layers of sodium = 2⨯10-9 / 1017 W = 2⨯10-26 J s-1 = 2⨯10-26 /1.6⨯10-19 = 1.25⨯10-7 eV s-`1
Since the work function of sodium is 2 eV, an electron will be ejected as soon as it gathers energy equal to 2eV. Therefore, the time required for photoelectric emission = 2 / 1.25⨯10-7 = 1.6⨯107 s ∼ 0.5 year
It is contrary to the observed fact that there is no time lag between the incidence of light and the emission of photoelectrons.
NCERT Textual Problems Unit-7-B
Q.7.31
Crystal diffraction experiments can be performed using x-rays or electrons accelerated through appropriate voltage. Which probe has greater energy? NCERT Textual Problems Unit-7-B
For quantitative comparison, take the wavelength of the probe equal to 1 Å, which is of an order of interatomic spacing in the lattice. Given mc = 9.11⨯10-31 kg.
NCERT Textual Problems Unit-7-B
Solution:
For X-ray photons of wavelength of 1 Å :
λ = 1 Å = 10-10 m
E = h c / λ = (6.62⨯10-34 ⨯ 3⨯108 )/ 10-10
= 1.986⨯10-15J
= 1.986⨯10-15 / 1.6⨯10-19 = 12.4⨯103 eV =12.4 KeV
NCERT Textual Problems Unit-7-B
For the electron of wavelength 1 Å:
λ = 1 Å = 10-10 m
Now, λ = h/m v or m v = h/λ = 6.62⨯10-34 / 10-10 = 6.62⨯10-24 Kg m s-1
The energy of the electron, E = 1/2 m v2 = (m v)2 / 2 m = (6.62⨯10-24)2 / 2⨯9.11⨯10-31 = 2.405⨯10-17 J = (2.405⨯10-17) / 1.6⨯10-19 =150.3 eV
It follows that X-ray photon has greater energy.
NCERT Textual Problems Unit-7-B
Q.7.32
(a) Obtain the de- Broglie wavelength a neutron of kinetic energy 150 eV. As you have seen in Q.7.31, an electron beam of this energy is suitable for crystal diffraction experiments. Would a neutron beam of the same energy be equally suitable? Explain. Given mn = 1.675⨯10-10 Kg.
NCERT Textual Problems Unit-7-B
(b) Obtain the de-Broglie wavelength associated with thermal neutrons at room temperature (27 o͘͘͘͘͘͘C). Hence explain why a fast neutron beam needs to be thermalized with the environment before it can be used for neutron diffraction experiments.
NCERT Textual Problems Unit-7-B
Solution:
(a) Here, m = 1.675 ⨯10-27 Kg ;
E = 150 eV = 150⨯1.6⨯10-19 = 2.4⨯10-17 J
Now, λ = h/m v = h / (2 m E)1/2
Therefore, a de-Broglie wavelength of neutron, λ = 6.62⨯10-34 /( 2⨯ 1.675 ⨯10-27⨯2.4⨯10-17)1/2 = 6.62⨯10-34 /2.835⨯10-22 = 2.335⨯10-12 m
Since the interatomic spacing (∼ 10-10 m) is about 100 times greater than the de-Broglie wavelength of 150 eV neutrons, they are not suitable for crystal diffraction experiments.
NCERT Textual Problems Unit-7-B
(b) Here, T = 27 +273 = 300 K
Energy of neutron at temperature T, E = 3/2 k T
Taking the mass of the neutron, m = 1.675 ⨯10-27 Kg, we have λ = 6.62⨯10-34 /( 2⨯ 1.675 ⨯10-27⨯6.21⨯10-21)1/2
= 6.62⨯10-34 / 4.56⨯10-24 = 1.452⨯10-10 m
Thus, thermal neutrons have a wavelength of the order of interatomic spacing (∼10-10 m) and therefore they are suitable for diffraction experiments. Fast neutrons will possess a wavelength quite small as compared to interatomic spacing and are, therefore, not suitable for diffraction experiments. Hence, for neutron diffraction experiments, a fast neutron beam needs to be thermalized.
NCERT Textual Problems Unit-7-B
Q.7.33
An electron microscope uses electrons accelerated by a voltage of kV. Determine that the de-Broglie wavelength is associated with the electrons. If other factors (such as numerical aperture, etc) are taken to be roughly the same, how does the resolving power of an electron microscope compare with that of an optical microscope that uses yellow light?
NCERT Textual Problems Unit-7-B
Solution:
Here, V = 50 k V
Therefore, the energy of electrons is E = 50 KeV = 50⨯103 ⨯1.6⨯10-19 = 8.0 ⨯10-15 J
Now, λ = h/ (2 m E)1/2
Taking m = 9.1⨯10-31 Kg, we have
λ =6.62⨯10-34 /( 2⨯9.1⨯10-31 ⨯8.0⨯10-15)1/2
= 6.62⨯10-34/ 1.207⨯10-22 = 5.485⨯10-12 m
The resolving power of a microscope is inversely proportional to the wavelength of the radiation used. Since the wavelength of the yellow light is 5,990 Å i.e. 5.99⨯10-7m, it follows that the electron microscope will possess resolving power 105 times as large as that of the optical microscope.
NCERT Textual Problems Unit-7-B
Q.7.34
The wavelength of a probe is roughly a measure of the size of a structure that it can probe in some detail. The quark structure of protons and neutrons appears at the minute length scale of 10-15 m or less. This structure was first probed in the early 1970s using high-energy electron beams produced by a linear accelerator at Stanford, USA. Guess what might have been the order of the energy of these electrons = 0.511MeV.
NCERT Textual Problems Unit-7-B
Solution:
As the quark structure of protons and neutrons appears at the length scale of 10-15 m, the electron beam used should be of the wavelength of this order i.e.
λ = 10-15 m
Therefore, the momentum of the electron beam,
p= h/λ = 6.62⨯10-34 / 10-15 = 6.62⨯10-19 Kg m s-1
The electron having momentum of this order possesses a speed comparable to the speed of light. Therefore, its energy can be found by using the relativistic formula for the total energy of the electron i.e.
E = (m0 2 c4 +p2 c2)1/2
Here, m0 c2 = 0.511 MeV
And p c = 6.62⨯10-19 ⨯ 3⨯108 = 1.986⨯10-10 J
= 1.986⨯10-10/1.6⨯10-13 = 1,241.25 MeV
Therefore E =[ (0.511)2 + (1241.25)2]1/2 = 1241.25 MeV
= 1.24 BeV
Q.7.35
Find the typical de Broglie wavelength associated with a He atom in helium gas at room temperature ( 27oC ) and 1 atm pressure, and compare it with the mean separation between the two atoms under these conditions.
NCERT Textual Problems Unit-7-B
Solution:
Given that, T = 27oC = 27 + 273 = 300 K
The mass of the He atom = At. wt. of He/Avogadro’s No = 4 gm/6.23 x 1023 = 0.642 x 10 -26 Kg. The relation between the average KE and the temperature of the Helium (1/2)mv2 = (3/2) kT => m2 v2 = 3 m kT => mv = (3 m kT)1/2
Also, λ = h/p = h/mv = h/(3 mkT)1/2 =6.62⨯10-34 /(3 x 0.642 x 10 -26 x 1.38 x 10 -23 x 300)1/2 = 0.73 ⨯ 10-10 m
NCERT Textual Problems Unit-7-B
Q.7.36
Compute the typical de Broglie wavelength of an electron in a metal at 27 o C and compare it with the mean separation between the two electrons in the metal, which is given to be about 2 ⨯10-10 m.
NCERT Textual Problems Unit-7-B
Solution:
Given that, T = 27oC = 27 + 273 = 300 K, h = 6.62⨯10-34 J s and me = 9.11 x 10 -31 Kg.
Using the relation for de Broglie wavelength of an electron: λe=h/(3mkT)1/2 = 6.62⨯10-34 /(3 x 9.11 x 10 -31 x 1.38 x 10 -23 x 300)1/2 = 6.2 x 10 -9 m The two electrons in a metal are separated by a mean distance of r = 2 ⨯ 10-10 m. Therefore, λ/r = 6.2 x 10 -9 m / 2 ⨯ 10-10 m = 31
This shows that the de Broglie wavelength associated with electrons at room temperature is much higher than the separation between them.
NCERT Textual Problems Unit-7-B
Q.7.37
Answer the following questions:
(a) Quarks inside protons and neutrons are thought to carry fractional charges (+2 e/3, -e/3). Why do they not show up in Millikan’s oil drop experiment?
(b) What is so special about the combination e/m? Why do we not simply talk of e and m separately?
(C) Why should the gases be insulators at ordinary pressure and start conducting at very low pressures?
(d) Every metal has a definite work function. Why do all the photoelectrons not come out with the same energy, if the incident radiations are monochromatic? Why is there an energy distribution of photoelectrons?
( e ) The energy and momentum of an electron are related to the frequency and wavelength of the associated matter wave by the relation:
E = hv ; p = h/λ
But while the value of λ is physically significant, the value of frequency v (and therefore, the value of phase speed v λ) has no physical significance. Why?
NCERT Textual Problems Unit-7-B
Solutions:
(a) Quarks having fractional charges are thought to be confined within a proton or a neutron only. These quarks are bound by forces, which grow stronger if they are tried to be pulled apart. Thus, the quarks always remain together. It follows that though fractional charges exist in nature, the observable charges are always an integral multiple of e, the charge of the electron in magnitude only. NCERT Textual Problems Unit-7-B
(b) The following equations represent the motion of an electron with in the electric and magnetic fields:
eV = (1/2) mv2 , eE = ma, and B ev = mv2 /r, where the symbols have their usual meanings.
It has been observed that charge and mass (e, and m) of an electron occur together in all the three equations given above and in none of them, e and m occur separately. It is also important to note here that the value of any physical variables appearing in any of the above equations can be found by substituting the value of (e/m).
(c) When the energetic rays like X-rays, cosmic rays etc pass through the gases, they continuously keep on ionizing. The positive and negative ions so produced are very close to each other at atmospheric pressure so that they can recombine to form neutral gas atoms. On account of this reason, the gases behave like insulators at ordinary pressure. But the ionized gas atoms, at low pressures start conducting. Thus, the gases behave as conductors at low pressures.

This resource is unbelievable. The radiant data exhibits the manager’s excitement. I’m shocked and anticipate more such astonishing presents.
This is an amazing page. The outstanding information reveals the owner’s accountability. I’m in awe and eagerly await more amazing postings like this one.
Hey, I’m Jack. Your blog is a game-changer! The content is insightful, well-researched, and always relevant. Great job!