Electric Cells, types and their Grouping-1
Introduction of Electric Cells, types and their Grouping-1
Volta, a scientist in 1800, with the idea that a continuous electric power can be generated from the fluids when used as conductor. After a long effort, the cell he discovered was named as a Voltaic cell. An electric cell mainly consists of three parts- Two metallic Electrodes, Electrolytic solution and a glass, plastic or a metallic container.
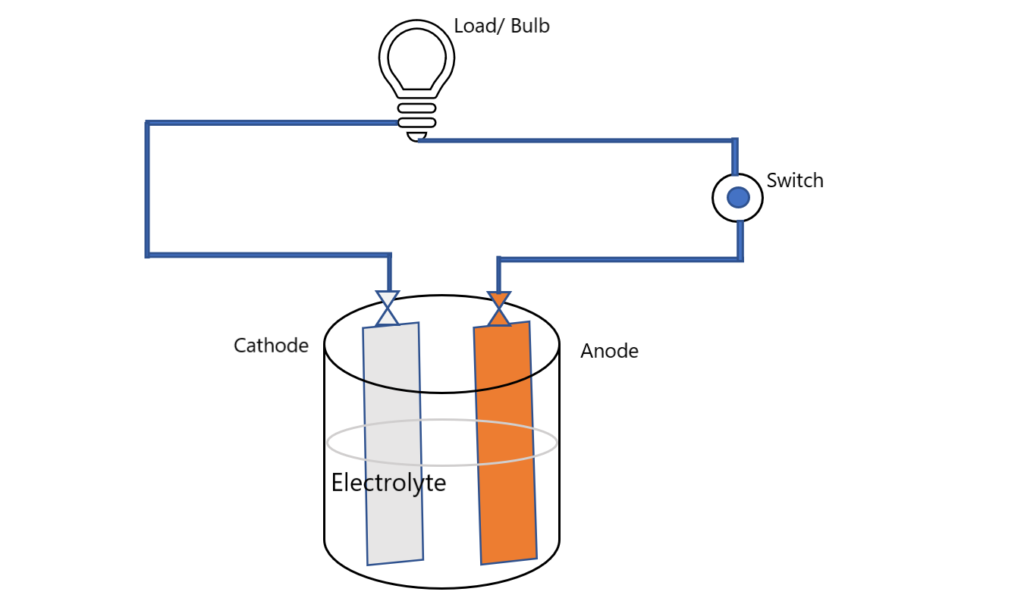
Electric Cells are the devices which converts chemical energy or any other type of energy into electric energy. Basically. Basically two metallic electrodes are immersed in an electrolytic solution like NaCl, CuSo4, etc. taken in vessel.
As a result of chemical reaction, the electrons are liberated and collect over an electrode called as cathode while the is called as an anode. When a load resistance is connected to these electrodes, a potential difference is applied to it and an electric current starts flowing through the load resistance.
Types of Cells -Electric Cells, types and their Grouping-1
There are several types of cells in use today depending upon the fact that which type of energy is being converted into electric energy. The electric cell which convert chemical energy into electrical energy are called as electro-chemical cell or voltaic cell. The another type is the photo voltaic cell or photo cell which convert solar energy into electric energy. It is also called as solar cell. Basically, the electric cells of all categories are divided in two types.
Primary cells: In primary cells, the chemical reaction is irreversible and hence these cells cannot be recharged by passing the electric current through their electrolytes after they have been discharged and are discarded permanently after their discharge. In primary cell. only chemical energy is converted into electrical energy. These cells have high internal resistance.
Secondary cells: These are the electric cells which after a long and repeated use get discharged and stop working, then these cells are recharged by passing an electric current through them. The chemical reaction that takes place in these cells is reversible These cells have low internal resistance
Comparison between Primary and Secondary cell-Electric Cells, types and their Grouping-1
| Primary cells | Secondary cell |
| In primary cells the chemical reaction is irreversible. | In secondary cells the chemical reaction is reversible |
| Chemical energy is converted into electrical energy. | Chemical energy is converted into electrical energy during energy supply and electrical energy into chemical energy with in the cell during charging process |
| They cannot be recharged | These types of cells can be recharged and are reusable |
| Internal resistance is high. | Internal resistance is low. |
| Their use is subjected to the supply of weak currents only. | They can be used for supply of weak and high currents. |
| Light, cheap and easy to handle | Secondary cells are heavy and high priced but long long life |
| Examples: Simple voltaic cell and dry cells | Examples: Lead, accumulators, acid batteries and Ni-Fe |
Internal Resistance and emf of the cell-Electric Cells, types and their Grouping-1
The internal resistance of a cell is defined as the obstruction by the electrolyte to the electric current when it flows through it.

When the cell woks, the electrons, bodily move from its -ve terminal to its +ve terminal through the circuit out side the cell but from its +ve to -ve terminal with in the cell through its electrolyte. In this way, the electrons has to move against the background of other ions and the neutral atoms within the electrolyte which appears in the form of the internal resistance of the cell to the flow of current. The internal resistance of freshly prepared cell is minimum while it goes on increasing with passage of time of its use.
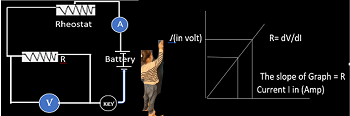
Expression of internal Resistance of a cell: Suppose that a cell of emf E is connected to an external resistance R through a key K and a high resistance voltameter is also connected in the circuit in parallel to the cell as shown below.

Now the circuit is kept open by not inserting key so that no current flows through the circuit, therefore, the reading of the voltameter as per definition of electro motive force will give us the emf of the cell. Let it be E.
Now key is closed so that an electric current I passes through the circuitry resistances r and R in series, then E= I(R+r) and current will be I = E/(R+r) —1 In this closed circuit conditions, the emf of the cell is the sum of the potential differences across the internal resistance r of the cell and the external resistance R. So that we can write the value of the emf as E= V + I r or V =E- I r —2
The terminal potential difference is defined as the potential difference across the external resistance R under closed circuit conditions, thus V=I R —3 From eqns. 1 and 3, V=[E/(R+r)]. R => R + r = (E/V). R Therefore, r=[(E/V) -1]. R This equation gives the internal resistance in terms of emf of the cell and the terminal potential difference
The emf of cell is abbreviated form of electro motive force which represents the amount of work done by it to drive a unit charge once round the circuit. Thus, emf = Work / charge and so emf = w/q The emf is measured in SI units as joule. coulomb-1 (JC-1 ) or in volt The emf of a cell is generally defined as the potential difference across its terminals in an open circuit conditions when no electric current is drawn from the cell.
Terminal potential difference of a cell:
The terminal potential difference of a cell is defined as the potential difference across the external resistance when the circuit is closed and the electric current I passes through the circuit.
Let us suppose that an external resistance R is connected across a cell of emf E volt having internal resistance r. When key K is closed, an electric current I passes through the circuit, then we can write the eq V = IR. The terminal potential difference is also equal to the emf of the cell under open circuit conditions when no current flows through the circuit.
Grouping of the cells and types-Electric Cells, types and their Grouping-1
A single cell supplies an electric current through a circuit in a limited amount on account of its capacity and limitations. But there are numerous electronic circuits where a greater amount of electric current is required. Therefore, two or more cells are connected in order to get the required amount of current. This process of connecting more cells together to meet the proper requirement of current is called as Grouping of cells. The grouping of cells is of the following three types.
When cells are of the same emf and internal resistance:
Let us suppose that n cells each of emf E and internal resistance r are connected in series with an external resistance R. In series combination, the negative terminal of the first cell is connected to the positive of the second, the negative of the second cell to the positive of the third cell, and so on.

Total emf of n cells each of emf E connected in series = nE The total internal resistance of the series grouping of n cells, each having internal resistance equal to r will be= nr, Therefore, the total resistance of this circuit will be = R + nr
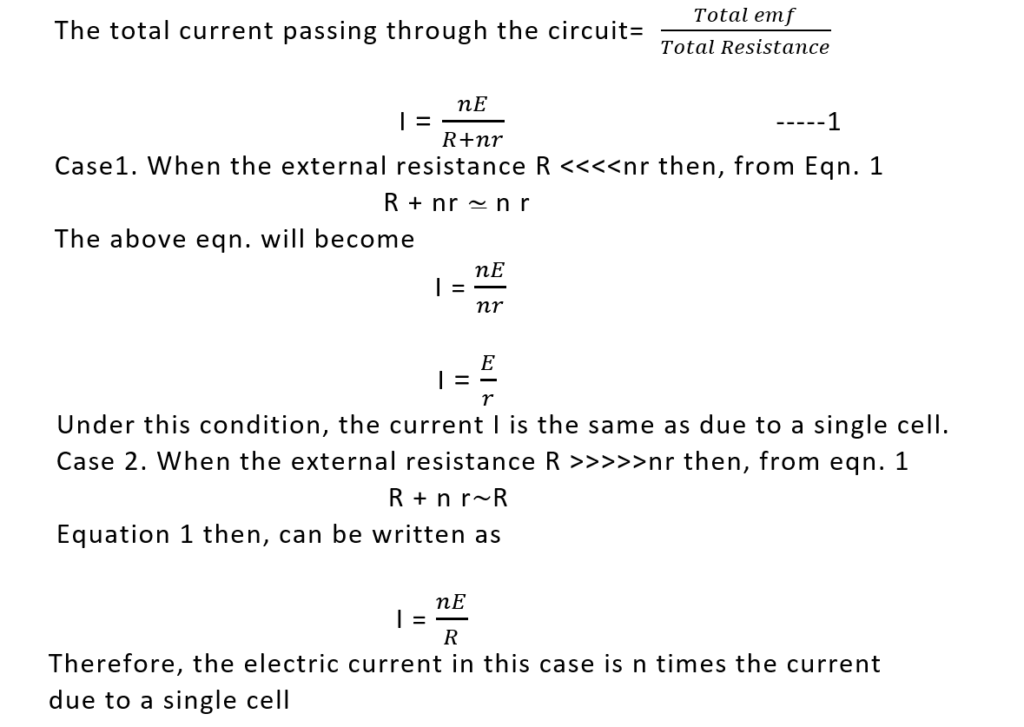
This is concluded that maximum electric current can be obtained by connecting a no. of cells in series under the condition that their internal resistances must be negligibly small as compared to the external load resistance R
When the cells are of different EMFs and internal resistances:



This gives the Electric current when the cells are connected in series.
Grouping of cells in parallel-Electric Cells, types and their Grouping-1
When cells are of same emf and internal resistance

Let us suppose that m cells each of emf E volt and internal resistance r be connected in parallel. Since the cells are connected in parallel, thus, the total emf of this grouping is equal to the emf of a single cell, E, and the total internal resistance of this parallel combination will be


When cells are of different emf and internal resistance:



Solving the above equation for the terminal velocity V, for the two cells (say) then, we get, V = (E1 r2 + E2 r1) / (r1 + r2) – I. [ (r1 r2)/ (r1 + r2)], Compare this eqn. with that of the relation between the terminal potential difference and the effective internal resistance of two cells, V = E -I r. Thus, we have, E = (E1 r2 + E2 r1) / (r1 + r2)and, r = (r1 r2)/ (r1 + r2) These boxed equations give us the effective emf and effective value of the internal resistance of the parallel combination of the cells
Mixed Grouping of cells-Electric Cells, types and their Grouping-1



Summary-Electric Cells, types and their Grouping-1
- The total emf of a series combination of cells is n times the emf of a single cell, and the total internal resistance of this combination is n times the internal resistance of each cell. ET = nE and rt =nr
- When R >>>> nr, then cells be connected in series to get maximum amount of current though the load resistance.
- In parallel combination of n cells having same E and r, then, the total emf of the combination will be equal to that of a single cell and the total internal resistance of this combination will be 1/n times that of a single cell.
- When the load resistance is negligible, the the cells are advised to be connected in parallel in order to have maximum current.
- Mixed grouping of cells is considered to get the maximum amount of current when the external load resistance is equal to the total internal resistance of the cells

2 Comments