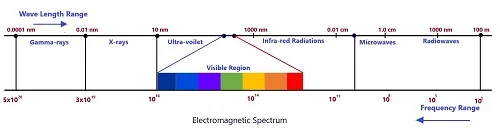
Electromagnetic Spectrum
This is an orderly distribution of electromagnetic waves, according to wavelength or frequency in the form of distinct groups.
what is an electromagnetic Spectrum?
Hertz and other scientists proved that it is possible to produce electromagnetic waves of wavelengths, ranging from several kilometers to 6 x 10-3 m. The visible light is found to be electromagnetic waves of very short wavelength produced by atoms and molecules, which behave as natural oscillators. By the end of last century, electromagnetic radiations, ranging from several kilometers to 6 mm and from 10-6 m (infrared) to 10-8 m (ultraviolet) became known.
Then, X- Rays were discovered, which were found to be electromagnetic waves of even shorter wavelength. The study of radioactive phenomenon led to the discovery of gamma rays, which are electromagnetic waves of wavelength, even shorter than that of X- rays. Then, originally existing gaps in the Electromagnetic spectrum got filled on the production of corresponding electromagnetic waves experimentally.
The orderly distribution of electromagnetic waves according to wavelength or frequency in the form of distinct groups, having widely differing properties is called Electromagnetic spectrum.
Electromagnetic waves are classified according to the type of the excitation. Overlapping in certain parts of the spectrum means that the corresponding wavelengths can be produced by two methods. For an example, 10-4 waves can be produced both with the help of an artificial oscillator and in the process of thermal radiations. It may be pointed out that the physical properties of electromagnetic waves are determined by the wavelength and not by the method of their excitations.
Main Components of Electromagnetic Spectrum
1 Gamma-Rays-Electromagnetic Spectrum
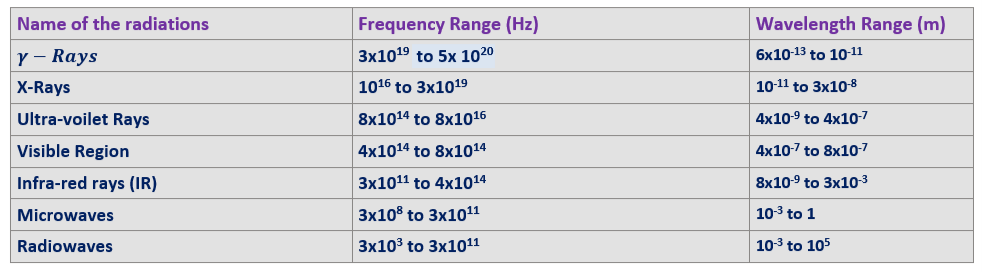
The gamma-rays are the high frequency radiations emitted mainly from the radioactive substances and so are highly energetic. These radiations are of nuclear origin and lie in the frequency range from 3 x 1019 to 5 x 1020 Hz. These radiations were discovered by Rutherford. They are the electromagnetic waves(in Electromagnetic Spectrum) of very short wavelength and can be detected with Geiger Muller counter.
Properties–
- Gamma radiations(in Electromagnetic Spectrum) are the high frequency electromagnetic waves and have velocity equal to that of light.
- Gamma rays have high penetrating power and so can penetrate iron and lead blocks up to several centimeters.
- When these radiations are allowed to pass through a an ionizable medium, it is observed that these rays ionize that medium to small extent as they have small ionizing power.
- They can produce fluorescence in a substance like Willamette.
- They can affect a photographic plate.
- Gamma rays are not the stream of charged particles but are the stream of high energy photons, therefore cannot be deflected by electric and magnetic fields.
- Gamma ray’s knockout the electrons from the surface the metals on which they are made to fall on.
- These radiations produce heating effects in the surface exposed to them. It means, the gamma-photon transfer the momentum as they strike the surfaces.
Applications of gamma rays
- Gamma rays are used in radiotherapy. In hospitals, they are used to treat cancer and tumors.
- In food industry, soft gamma radiations are used to kill microorganism. It helps to preserve the food stuffs for a prolonged time.
- Gamma rays are used to produce nuclear reactions.
2. X-rays-Electromagnetic Spectrum
X Rays were discovered accidently in by the German physicist W Rontgen. These rays are of atomic origin and are produced when a target of an element having high atomic number (high z- value) is bombarded with the energetic cathode rays (fast moving electrons). X Rays have the frequencies in the range. 1016 to 3 x 1019 Hz. X Rays possess a high penetrating power. This component of Electromagnetic Spectrum is very important from its application point of view.
Properties
- X-rays are the electromagnetic waves of very short wavelength, ranging from 0.01 A0 to 10 A0 which is comparable to the size of the atoms in the crystals. Therefore, the X-rays can be diffracted easily from the crystals when incident upon them.
- X rays travel in vacuum with the speed of light 3 x 108 m/s as they are also the electromagnetic waves.
- X-rays are not the stream of charged particles but are the stream of high energy photons, therefore, they cannot be deflected by electric and magnetic fields.
- They can affect a photographic plate very intensely.
- The ionize the gasses easily through which they pass.
- They can produce fluorescence in a substances like zinc sulphide, Barium platino-cyanide, calcium tungstate, etc.
- X-rays, similar to light, can also produce photoelectric effect.
- They travel in straight line and while doing so, they cast the shadows of the objects falling in their path.
- X-rays can produce a reflection, refraction, interference, deflection, and polarization similar to that as light does in optics.
- X-rays can penetrate the materials that are opaque to visible or ultraviolet light. They can easily pass through paper, thin sheet of metals, wood, flash, etc. but they cannot penetrate denser objects such as bones, heavy metals, etc.
- These radiations have injurious effect on human bodies. Exposure of human body to X Rays, causes reddening of the skin. The long exposures result into surface sores
- When the X ray fall on certain metals secondary, X-rays are produced, which are characteristic of the metal. The secondary, X Rays are accompanied by fast moving electrons.
Applications of X-rays
- Surgery: X Rays are successfully used in surgery for the detection of fractures, deceased, organs, foreign matter like bullets and formation of bones or stones in the human body and observing the progress of healing bones. They are also used to diagnose the diseases. Advance technology-based x-rays have provided an excellent facilitation in the field of surgery.
- Radiotherapy: Controlled X- rays’ exposures are used to cure untraceable skin diseases and malignant growths. Soft X- Rays are used if the affected parts are superficial. While hard X- rays are used for deep seated organs.
- Engineering: X-rays are used in engineering for detecting faults, cracks, flaws and gas pockets in finished metal products. They are used for the testing of welding’s, castings and moulds. They are also used to detect cracks in the engines of cars and airplanes.
- Detective Departments: They are used by detective departments for detection of explosives, opium and other contraband goods, and are used by Custom Department for detecting gold and silver in the body of smugglers. They are also used in mints where the coins are made. They are also used for distinguishing real diamonds, gems, etc. from artificial ones.
- Industries: X- rays are used in industry for detecting the pearls in oysters, for testing the uniformity of insulating materials and homogeneity of timber. They are also used to examine the defect in rubber tyres, golf and tennis balls, woods, etc.
- Scientific Research: X-rays have been used in investigating the structure of the crystals, constitution and properties of atoms and arrangement of atoms and molecules in the complex substances. In addition to the above mentioned uses, the X Rays are also used in detecting changes in old oil paintings.
3. Ultra-violet-Rays:(in Electromagnetic Spectrum)
Ultra-violet-Rays were discovered by Ritter in 1801. The Ultraviolet-rays are the part of solar spectrum. They can be produced by the arcs of mercury and iron. They can also be obtained by passing discharge through hydrogen and xenon. The frequency of ultraviolet rays lies in the range of 8 x 1014 to 8 x 1016 Hz.(in Electromagnetic Spectrum)
Properties:
- Ultraviolet rays travel in vacuum with the speed of light 3 x 108 m/s in air or vacuum as they are also the electromagnetic waves.
- They also follow the laws of reflection and Refraction similar to that as light in optics.
- They can be polarized and can produce interference when the two coherent beams of ultraviolet rays are made to pass through a medium.
- They cause the emission of photoelectrons from the metals when allowed to fall on their surfaces under suitable conditions
- They can affect the photographic plates like most of the electromagnetic waves. (In Electromagnetic Spectrum)
- They can produce fluorescence in certain substances like zinc sulphide, Barium platino-cyanide, etc.
- Ultraviolet ray cannot pass through glass easily pass through quartz, fluorite, and rock salt as if these materials are transparent to these rays.
- Ultra-violet rays efficiently capable of synthesizing vitamin D when the skin gets exposer to the sunlight.
Applications of Ultraviolet Rays
- Now a days, the ultraviolet rays are being used on a large scale for checking the mineral samples due to its property of causing the fluorescence.
- These radiations are used in the process of sterilization of surgical instruments and to destroy the bacteria in food stuffs.
- As the ultraviolet rays can produce the photoelectric effect, so they can be successfully used in burglars alarm.
- The absorption spectra of ultraviolet radiations are used to study the molecular spectra.
4. Infra-red Rays (in Electromagnetic Spectrum)
Infrared radiations were discovered by Herschelle. Infrared rays are the heat radiations and therefore all hot bodies are the source of infrared rays. About 60% of the solar radiation is infrared in nature. The frequency range of infrared rays is 3 x 1011 to 4 x 1014 Hz. These radiations can be produced for laboratory and for other purposes using the following sources
- Nernst Lamp: The filament of nest lamp is made from the mixture of zirconium thorium and cesium. When a current is passed through such a filament, then at a temperature of about 1200 K the Infrared rays are emitted.
- Globar: It is basically a rod of silicone carbide which when heated to a temperature of about 900 K by passing an electric current through it, the infrared radiations are produced.
- LASER: The principle of light amplification by stimulated emission of radiations is employed to produce the monochromatic infrared radiations. For example, C O2 LASER provides infrared rays of wavelength 10.6 x 10-6 m, while He-Ne laser gives infrared rays of wavelength 0.69 x 10-6 m, 1.19 x 10-6 m and 3.39 x 10-6 m. The several equipment’s are utilized to detect these radiations which includes, thermocouples, thermopiles, photo conducting cells and bolometers, etc.
Properties of Infrared rays(in Electromagnetic Spectrum)
- Infrared rays travel in vacuum with the speed of light 3 x 108 m/s in air or vacuum as they are also the electromagnetic waves.
- They also follow the laws of reflection and Refraction similar to that as light in optics.
- They can be polarized and can produce interference when the two coherent beams of ultraviolet rays are made to pass through a medium.
- Infrared rays produce heat in the matter and increase their temperature when allowed to fall on the matter.
- They can affect the photographic plates like most of the electromagnetic waves.
- Infrared rays produce molecular vibrations as their energy is absorbed by the molecules.
- They are scattered less in comparison of the visible light by the atmosphere; hence the Infrared rays can travel through longer distances through the atmosphere under the conditions of smoke, fog, etc. Nitrogen and oxygen gasses are found to be transparent medium to all the wavelengths of infrared rays.
Applications of Infrared Rays(in Electromagnetic Spectrum)
- Infrared rays from the sun keeps the earth warm and hence helps to sustain life on the Earth.
- The coal deposits in the interior of earth are the result of conversion of forest woods into coal, due to infrared rays.
- Infrared wheels are used in solar water heaters and cookers. (in Electromagnetic Spectrum)
- Infrared leaves photographs are used for weather forecasting. (in Electromagnetic Spectrum)
- Infrared rays are used for taking photographs during the conditions of fog smoke, etc.
- Infrared rays’ absorption spectra is used in the study of the molecular structure and then to check the purity of the chemicals.
- Infrared rays are used for producing dehydrated fruits. (in Electromagnetic Spectrum)
- Infrared rays are used to provide electrical energy to satellites by using solar cells and also used to treat muscular strains
5. Microwaves and Radio waves (in Electromagnetic Spectrum)
The microwaves are produced by oscillating electronic circuits. The frequency of microwaves lies between 3 x 108 and 3 x 1011 Hz. The microwaves are used in radar and other communication system. The microwaves have comparatively smaller wavelength range. The radio waves are also produced by oscillating electronic circuits having an inductor and a capacitor in parallel. The frequency of radio waves lies between 3 x 103 and 3 x 1011 Hz. The radio waves are used as carriers’ waves in the radio broadcasting and TV transmission systems.
Properties of Microwaves and Radio waves (in Electromagnetic Spectrum)
- Microwaves and Radio waves, both are the electromagnetic waves in character and travel with speed equal to that of light in vacuum or in air (3 x 108 m/s).
- The radio waves obey the laws of reflection and the laws of refraction as in optics
- The radio waves are of the wavelengths comparable the size of the atoms in the crystals obeying the basic required condition for the diffraction. Thus, the radio waves get diffracted from the obstacles coming in their path.
- Microwaves produce h eat when absorbed by the matter
Applications of Microwaves and Radio waves (in Electromagnetic Spectrum)
- Radio waves are used for wireless communication systems and are used for the transmission of radio and TV signals.
- Radio waves are used in the field of radioastronomy.
- In the field of radio detection and ranging, (RADAR system) the microwaves are playing a significant role.
- Microwaves are used in long distance transmission in case of telephone communication systems.
- The microwave ovens used in cooking’s are based on the principle of Microwaves.
- Microwaves are used in the study of atomic and molecular structures of the solids and crystals.
6. Visible Region (in Electromagnetic Spectrum)
It forms a very narrow part of the electromagnetic spectrum and its frequency to ranges from 4. To 8, the visible light is emitted due to the atomic excitations. Human eye is sensitive to only visible part of the electromagnetic spectrum.