Photoelectric effect-2
This part of Photoelectric effect-2 describes the determination of Plank’s constant and work function of the metals.
It also describes the relation between cut off potential, frequency of incident photon and threshold frequency/wavelength, Failure of wave theory of light to explain photoelectric effect and photon theory of light to explain photoelectric effect e.t.c,
Determination of plank’s constant and work function
As discussed in Photoelectric effect under sub head, Einstein’s photoelectric equation, the maximum kinetic energy acquired by the photoelectrons is given by

If Vo is the stopping potential or cut off potential, then, (1/2) mv2max = eV0 – – (ii) Now comparing Eqn. (i) and (ii), we get, eV0 = h (v – v0) = hv – hv0 = hv – W0 => V0 = hv/e + (-W0)/e – – – -(iii) when this equation (iii) is compared with the equation of a straight line, y = mx + c – – – -(v), where m is the slope of the line its intercept with the y – axis is c, therefore, the graph between the stopping potential V0 and the frequency of the incident radiations v is a straight line as shown in [Fig. 11.08]. (Photoelectric effect-2)


Using eqns. (v) and (vi), the values of Plank’s constant and the work function of a metal can be found and R. A. Millikan was the first to measure the values of Plank’s constant and the work function W0 for sodium. (Photoelectric effect-2)
Relation between cut off potential Frequency and threshold Frequency
The maximum kinetic energy gained by a photoelectron, according to Einstein’s photoelectric equation is given by Kmax and thus,

Also, we can write Kmax = eV0 and W0 = hv0 Putting these in the equation (i) given above, eV0 = Kmax = hv – hv0 = h(v – v0)

The above given equation provides the required relation between the cut off potential, frequency of the incident photon and the threshold frequency or the wavelength. (Photoelectric effect-2)
Failure of wave theory of light to explain photoelectric effect
Light behaves like electromagnetic waves that consists of electric and magnetic field, according to the Huygen’s wave theory. The electric and magnetic fields vary sinusoidally in the directions perpendicular to each other and perpendicular to the direction of propagation of wave. The energy is distributed continuously over the region up to which the wave extends. The following are the reasons which describe the reasons of failure of Huygen’s wave theory of light to explain the photoelectric effect. (Photoelectric effect-2)
- A beam of light, when, strikes a metal surface, the according to the wave theory of light, the free electrons at the surface of the metal absorbs this energy of wave continuously. It is therefore, evident that more the intensity of incident radiation, more will be the amplitudes of electric and magnetic fields and hence, more will be the energy density of the wave. Therefore, conclusively, higher intensity wave should emit the energetic photoelectrons with higher and higher energy values. But this hypothesis strongly contradicts the experimental result that the maximum kinetic energy of the emitted photoelectrons is independent of the intensity of the incident radiations. Therefore, the wave theory of light could not explain the photoelectric effect. (Photoelectric effect-2)
- According to the Huygen’s wave theory of light, the photoelectrons can be easily emitted from the metal surfaces, when the waves of light of all frequencies and of sufficiently higher intensities are made to incident over these metallic surfaces. Thus on increasing the intensity of incident wave, the energy can be increased and the photoelectric emission will be initiated even when the frequency of the incident wave is less than the threshold frequency of the photosensitive metal emitter. But this is in absolute contradiction the experimentally established law of photoelectric Effect that the photoelectric emission of the electrons from the metal surfaces is not possible, if the frequency of the incident light is less than the threshold frequency for the photosensitive metal irrespective of its intensity. (Photoelectric effect-2)
- When the light wave is made to strike the metal surface, the energy of the light wave is absorbed by the large number of electrons as the energy of light wave is smoothly and evenly distributed across its advancing wavefront and so, the energy absorbed per electron will be very small. Therefore, each electron requires some time to acquire sufficient energy equal to or more than the surface barrier energy in order to come out from the surface of metal. Thus, the delay of time will come between the incidence of light and emission of photoelectrons. This concept is against the experimental fact that the photoelectric emission is an instantaneous process and it takes place as soon as the light is incident on the metal surface. (Photoelectric effect-2)
Particle nature of light (the photon)
The experimental observations on photoelectric effect reveal a significant inference that when the light interacts with matter, it behaves as a stream of energy particles or the energy packets, each of energy hv, where h and v be the Plank’s constant and the frequency of the light respectively. These packets of energy are called photons. The photons being the energy particles, possess the momentum similar to as the matter particles have and is given by (hv/c). A finite value of energy and momentum provide an informatic indication that the light quantum acts as a particle called photon. The particle nature of light was confirmed by the experiment of scattering of X-rays by electrons performed by A.H. Compton. (Photoelectric effect-2)
The characteristic Properties of Photons:
(1) During the interaction of radiations (energy) with matter, the radiations behave as a stream of particles called protons.
(2) The energy and momentum possessed by each photon be E = hv = h c/ (lemda) and p = hv/c =h/(lemda), where, the letters have their usual meanings. (Photoelectric effect-2)
(3) A light photon travels in vacuum with a velocity equal to that of light (3 x 108 m/s). (Photoelectric effect-2)
(4) A photon can not exist at rest, since its rest mass is zero (m0 =0). According to Einstein’s theory of relativity, the mass of a particle moving with a velocity v which is comparable to the velocity of light c, is given by

Since the rest mass of the photon is zero, therefore, a photon can not exist at rest. (Photoelectric effect-2)
(5) The wavelength of the light photons changes from medium to medium, therefore, its velocity is different in different media.
(6) The electric and the magnetic fields do not exert force on the photon and hence can not be deflected since the photon is electrically neutral carrying no charge.
(7) All the photons of a particular frequency (v) possess the same energy, E = hv and same momentum, p = hv/c, irrespective of the intensity of the light radiations. (Photoelectric effect-2)
(8) When the light photon enters from one medium to anther medium, its frequency does not change. (Photoelectric effect-2)
(9) The equivalent mass of a photon, according to the Einstein’s mass energy equation, is given by E = mc2 = hv => m = hv/c2.
(10) The photon may exhibit the phenomenon of the diffraction of light under the given conditions.
(11) In the interaction of energy and matter, when photon collides with a matter particle, (such as in photoelectric emission) the energy and the momentum, both remain conserved. However, the number of photons may not be conserved in such types of collisions. One new photon may be created or one photon may be absorbed. (Photoelectric effect-2)
(12) If a source of light emits n photons per second each of frequency v, then the power of light radiated out by the source of light will be given as, Power = Energy/time = n (hv)/1 = n (hv) here, h = Plank’s constant.
Photoelectric cell
A photoelectric cell is a device which converts light energy in to electric energy. It is also called as an electric eye and it can easily measure the intensity of light by measuring the photoelectric current.
Types of Photoelectric cell
Photoelectric cells are of three types: (1) Photoemissive cell (2) Photovoltaic cell (3) Photoconductive cell
(1) Photoemissive cell:
Principle: This device works on the principle of Photoelectric Effect or photoelectric emission.
Construction:
It consists of the two electrodes, a cathode C and an anode A fitted in a highly evacuated glass bulb or a quartz bulb as shown in [Fig.11.09]
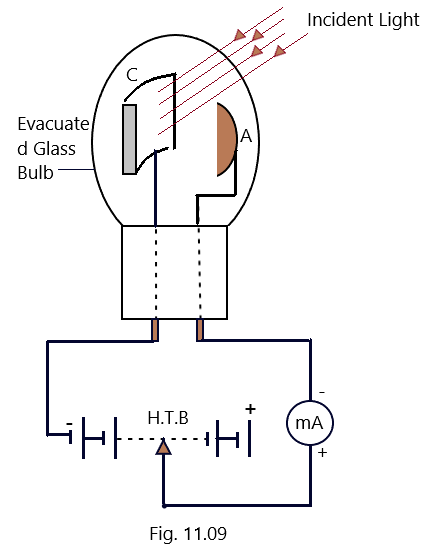
The cathode C, is coated with a photosensitive material like sodium (Na), potassium (K), cesium (Cs), and rubidium (Rb) and is a semi-cylindrical plate. A thin layer of photosensitive material is sometimes coated on the inner wall of the bulb in order to increase the efficiency and stability of the Photoemissive cell. (Photoelectric effect-2)
The anode A in the form of semi circular wire made of nickel (Ni) or platinum (Pt), is fixed in the bulb just opposite to the cathode so that it does not obstruct the path of the incident radiations and the photoelectron capture cross-section may increase. It is also called as collector as it collects the photoelectrons emitted from the cathode. Both of these electrodes are connected externally to a high tension battery (H. T. B.) and a micrometer.(Photoelectric effect-2)
Working of Photoemissive cell:
When a light radiation of frequency more than the threshold frequency for the photosensitive material of the cathode, is made to incident on the cathode, the photoelectrons are emitted which are attracted towards the anode and and are collected there and constitute a small current and is measured with the help of micrometer connected in the external circuit. As the number of photo electrons emitted from the cathode is proportional to the intensity of the incident radiations, therefore, the photoelectric current will be the measure of the intensity of light and so, the photocell converts a change in thein the intensity of incident radiations in to the change photo electric current. (Photoelectric effect-2)
This type of photocell has a very an important advantage that there is no time-lag between the incident radiation and the emission of photoelectrons and the photoelectric current so produced is found to be proportional to the intensity of light radiations made to incident on the cathode. This fact makes the Photoemissive cell, most accurate in response. Therefore, this type of cell is widely used in television and photometry. When the tube is filled with an inert gas at a pressure of a few mm of mercury, the photoelectric current is increased by a factor of 5 as revealed by the researches. (Photoelectric effect-2)
It is also observed that the photoelectrons emitted from the cathode, ionize the gas atoms, when the potential difference applied between the electrodes (cathode and anode) exceeds the ionization potential of the gas and at this stage, a much higher current flows in the external circuit. Such type of photocells are called the gas-filled photocells and are prohibited to use for registering of very fast changing illuminations due to the reason that it takes only 10-4 second for the ions to recombine and so the changes can not be followed in such a short interval of times. The current remains no longer proportional to the changing intensity of light. (Photoelectric effect-2)
On the other side, the gas -filled photocells are better to use in film projectors because it can respond to the changes in the intensity of light from the sound track. (Photoelectric effect-2)
(2) Photo conductive cell:
This type of the photocell makes use of the fact that the electric conductivity of the semiconductors increases with increase of in the intensity of incident light and so is a light-sensitive semiconducting device. Cadmium sulphide (Cd-S) and cadmium selenide (Cd-Se) are the materials that are normally used in photoconductive cell manufacture. When the voltage is applied across the cell it is found to vary with the light intensity. The conductivity of the cell decreases with no illumination, and increases with illumination. (Photoelectric effect-2)
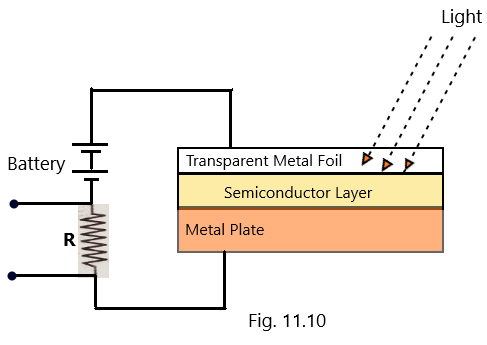
The photoconductive cell is also called as a photoresistor or light-dependent resistor and it works as per the principle of photoconductive effect which is a process in which the conductivity of a semiconductor material changes in accordance with the changes in the wavelength and intensity of the light radiation.
Construction and Working
In the circuit as shown in [Fig. 11.10], the resistance of the photoconductive cell is in series with an external resistance (R) which limits the current to the desired value when its value is suitably selected. A bias voltage is also used with the help of the battery connected in series with the photoconductive cell. (Photoelectric effect-2)
When the light radiation is not made to incident, the resistance of the photoconductive cell is very high and therefore, the current as measured using an ammeter is also very low which is called as dark current and the corresponding resistance is called as the dark resistance. This lowering in current due to the absence of light causes a voltage drop across the load resistor R. (Photoelectric effect-2)
When the light radiation is allowed to incident, the resistance of the photoconductive cell decreases, which results a large current to flow known as the joint current. This is due to the reason that the light energy supplied to the photoconductive cell leads its covalent bonds to be broken, hence the electron-hole pairs are created. On account of this increase in the number of charge carriers, the resistance of the material is proportional to the number of charge carriers. (Photoelectric effect-2)
(3) Photovoltaic Cell
The photovoltaic cell works on the principle that the potential difference can be developed between the suitable substances due to the photoelectric emission. An electric current is supplied by this type of cell with out using an external battery. It is widely used in the exposure meters of good quality cameras and in solar batteries. (Photoelectric effect-2)
Applications of photoelectric cells
- Automatic Fire Alarm: In sensitive places and fire prone area, a photoelectric cell is installed in conjunction with some suitable circuits. The light falls on the cell as the fire breaks out in its vicinity resulting in to the photoelectric emission. The photoelectrons so emitted initiate the electric current in the circuit and the bell starts ringing connected to the photo cell.
- Automatic Burglar Alarm: U.V radiations are made to incident continuously on the photo electric cell from a ultraviolet source. When a person (thief or burglar) crosses the beam of ultraviolet radiations, the radiations get blocked for the moments, the person crosses the path of the beam. As a result , the photoelectric current will undergo a sudden change initiating the electric bell to ring up indicating the entry of an un-authorized person.
- Photo Counting: The photoelectric cell has an important role in the working and the operation of a counting machine. The photoelectric current stops when a person or an object comes in the path of the light beam incident on the photoelectric cell resulting the activation of counting machine. Therefore, one by one, the entry of all the persons is successfully recorded.
- Cinema: The reproduction of the sound in the cinema halls is achieved using photoelectric cell.
- Automatic Furnace: The photocells are found of greater use to maintain the temperature of the furnace at a constant and desired level. When the temperature of the furnace increases from the desired level the radiations falling on the cell increases and so the photoelectric current also increases and the relay comes in to action putting the switch to off position. Similar is the case when the temperature of the furnace decreases below the require level, the relay operates the switch to on – position, and again the current supply is started to the furnace
